- Home
- Academics
- Faculty Research Initiatives
- Neuroscience Research Group
Current basic and clinical neuroscience research conducted within St. John’s College of Liberal Arts and Sciences aims to shed light on the multiple neural processes of normal and pathological behaviors.
The study of the nervous system and its relationship with body, mind, and behavior requires a multidisciplinary, collaborative enterprise to unravel the complex anatomical, physiological, and cognitive processes underlying behavioral manifestations. Current basic and clinical neuroscience research conducted within St. John’s College of Liberal Arts and Sciences aims to shed light on the multiple neural processes of normal and pathological behaviors.
Current Research
Dr. Christoforou’s research lies at the intersection of machine learning and neuroscience. In particular, his work focuses on the development of novel machine-learning approaches that analyze electroencephalography (EEG), heart rate, and eye-tracking data to characterize the neural-underpinnings of cognitive disorders. Moreover, he explores the use of such methods towards the development of neurotechnology solutions for application in domains such as brain-computer interfacing, neural-based predictive modeling, neuro-cinematics, human-robot interaction, and medical diagnostic systems. His recent research contributions include the development of machine-learning-based methods for the study of the neural correlates of imagined perspective-taking in 3D virtual environments; the study of neural-underpinnings of reading disorders during free-reading tasks; methods for EEG-based emotion recognition during emotional video viewing; and the extraction of neural-components during the viewing of video stimuli that predict population-wide preferences. Dr. Christoforou’s lab is equipped with two research-grade EEG and eye-tracking units, as well as a humanoid-robot that facilitates the experimental design, data collection, and the development of neurotechnology solutions.
To read more about Dr. Christoforou’s research, view his faculty profile.
Dr. Deshpande is a nationally-accredited audiologist whose research investigates the use of a variety of hearing testing procedures (including the use of “brainwaves”) for studying typical versus atypical listeners. She collaborates with national and international researchers for her auditory neuroscience projects and studies different populations, like adults and children with typical hearing abilities, individuals with cochlear implants and professionally trained musicians. This research has implications for the development and implementation of better intervention protocols for individuals with impaired auditory perception and processing.
To read more about Dr. Deshpande's research, view her faculty profile.
Francis Fallon is Associate Professor of Philosophy, with a joint appointment in Psychology, at St. John’s University in New York City. Currently he is the Project Director of Change Detection During Saccades, and a contributing member of the COGITATE Consortium. Both projects use empirical methods to test different theories’ competing predictions (“adversarial collaboration”), and are funded by the Templeton World Charities’ Accelerating Research on Consciousness initiative. He founded and co-directs the project Representation: Past, Present, and Future, supported by the Wellcome Trust Institutional Strategic Support Fund as part of Trinity College Dublin’s Neurohumanities program. He has published in PLOS One, Entropy, The Review of Philosophy and Psychology, Topoi, and the International Journal of Philosophical Studies, among other places. He also edited (with Gavin Hyman) Agnosticism: Exploration in Religious and Philosophical Thought (Oxford UP, 2020).
For more on his research, read his faculty bio.
Dr. Jackson’s research focuses on the neural correlates of passive and active musicking and the cognitive traits of musicians of varied musical backgrounds. Musicking — the act of engaging with music as a listener or performer — offers a unique and integrative way to investigate the coordination among different internal neurocognitive systems with external systems of musical understanding, including: music theory, dance, and therapy. As a professional musician and cognitive neuroscientist, he focuses on developing projects that address the interests of musicians and neuroscientists alike.
His current research focuses on the dynamic coupling and decoupling of selective attention and working memory during effortless and effortful musical improvisation. Building on prior music improvisation research which underscores the significance of mind-wandering and working memory in the improvisational process (Limb & Braun, 2008; Norgaard et al., 2016), his current research questions the internal dynamics of shifting attention; how these dynamics influence the capacity and duration of working memory; and to what extent the external musical structures influence these internal dynamics.
Past research projects include:
- Using the N2c/P3a ERP complex as an index of understanding among musicians with varying levels of improvisation experience
- Reevaluation of syntax ERPs using varied musical chord progressions in a passive listening environment
- Investigating how much familiarity with chord hand shapes influence guitarists’ perception of sonic congruence.
To read more about Dr. Jackson's research, view his faculty profile.
References
Limb, C. J., & Braun, A. R. (2008). Neural substrates of spontaneous musical performance: An fMRI study of jazz improvisation. PLoS one, 3(2), e1679.
Norgaard, M., Emerson, S. N., Dawn, K., & Fidlon, J. D. (2016). Creating under pressure: Effects of divided attention on the improvised output of skilled jazz pianists. Music Perception: An Interdisciplinary Journal, 33(5), 561-570.
Dr. Møller's research focuses on Parkinson's disease with an emphasis on defining molecular and cellular mechanisms associated with disease onset and progression and discovering new diagnostic and prognostic biomarkers for the disease. Dr. Møller's laboratory uses a combination of molecular biology, cell biology, protein biochemistry and bioinformatics. As many of the research projects have direct clinical relevance to patients Dr. Møller’s laboratory has multiple productive collaborations with health care institutions, including Dr. Joanne Donoghue at The Adele Smithers Parkinson’s Disease Treatment Center in the College of Osteopathic Medicine at New York Institute of Technology.
To read more about Dr. Møller's research, view his faculty profile.
Dr. Peckins investigates how youths’ social and physical environments become biologically embedded to impact vulnerability for health and behavior problems from childhood through adulthood. Specifically, Dr. Peckins studies the impact of different types of adversity (e.g., maltreatment, socioeconomic disadvantage) on neurobiological functioning, health, and behavior from a multisystem perspective. Dr. Peckins uses an interdisciplinary approach and incorporates behavioral measures, salivary and hair biomarkers, neuroimaging, and advanced analytic approaches in her research.
To read more about Dr. Peckins's research, view her faculty profile.
RNA Splicing in Neurons
The central nervous system comprises the tissues and cells with the highest rate of alternative splicing in the body, and RNA-binding proteins play a major functional role in neurons in both normal conditions and in disease. To better understand the contribution of RNA processing to nerve cell biology, and to help elucidate the function that RNA processing regulators play in neuron physiology and neurologic disorders, it is necessary to identify which RNA-binding proteins are involved in these biological pathways, and to characterize how they work at the molecular level.
Dr. Ruggiu’s long-term goal is to understand the molecular mechanisms regulating protein-RNA networks that control alternative splicing, and how they relate to neuron biology, and to disease of the nervous system. This research is funded by a grant from the National Institutes of Health (NIH).
To read more about Dr. Ruggiu's research, view his faculty profile.
The Vázquez group is interested in using multiscale molecular modeling to understand how protein oligomerization influences neurotransmission or neuronal protein aggregation. Our group focuses on two proteins: dynamin and α-synuclein. Dynamin oligomers are involved in membrane fission that allows the neuron to recycle the machinery used for neurotransmission. α-synuclein protein aggregates are one of the key hallmarks of Parkinson’s disease. Our group is interested in understanding how oligomerization leads to large-scale changes in the neuron and how those changes are influenced by molecular-level interactions. We use and develop multiscale modeling methods that are able simulate large protein oligomers while still taking into account the underlying molecular-level processes. This multiscale view gives us an understanding of how small-scale molecular interactions lead to very large changes in the cell.
To read more about Dr. Vázquez's research, view his faculty profile.
Dr. Wagner collaborates with neuroscientist Dr. Mitchell Steinschneider, neurolinguist Dr. Valerie Shafer, and hearing scientist and audiologist Dr. Brett Martin on these and other projects that examine auditory cortical processing of speech in English, Spanish, German and Russian speakers. She also collaborates on research examining sensory processing within the auditory cortex in infants and, with Dr. Jungmee Lee, is currently working on the development of computer scripts for large scale data analysis.
Recognition of Spoken Words within the Auditory Cortex
Dr. Wagner conducts research to uncover brain mechanisms that allow spoken words to be recognized for comprehension within the auditory cortex. Each spoken word that travels into our ears as sound waves contains unique characteristics. These characteristics are transmitted to high levels of the brain within the auditory cortex. Dr. Wagner’s research has identified brain wave patterns within the electroencephalogram (EEG), recorded from the scalp surface, that reflect recognition of spoken words (Wagner et al., 2016). Her research shows that these brainwave patterns do not change when modifying levels of attention (Wagner et al., in preparation) and has identified normal patterns of variability for brain responses to naturally spoken words (Wagner et al., under revision). Her research is now directed towards determining the way individuals with speech and language impairment, auditory processing disorder, dyslexia, and autism recognize spoken words for comprehension within the auditory cortex.
Native-Language Patterns of Speech Perception
Individuals who learn a second language as an adult have difficulty pronouncing non-native sounds. It is less commonly known, however, that these individuals also have difficulty hearing these non-native sounds. Dr. Wagner examined native-Polish and native-English listeners and found that Polish listeners could distinguish words that began with “pt” versus “pet,” a contrast that occurs only in the Polish language, whereas English listeners could not. She found that late stage cortical processing of speech reflected these behavioral patterns (Wagner et al., 2012) and early stages reflected the physical characteristics of the stimuli (Wagner et al., 2013). Her current research manipulates attention to determine whether brain wave patterns at intermediate stages reflect a transition from physical processing to language-specific processing.
To read more about Dr. Wagner's research, view her faculty profile.
Determining Cell Death Pathways Activated in ALS Models
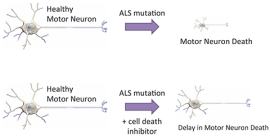
The purpose of Dr. Yang’s research is to explore cell death pathways in human disease context using genomics, small molecules, and metabolomic tools. The immediate goal is to characterize cell death pathways in degenerating motor neurons in amyotrophic lateral sclerosis (ALS) models. ALS, also known as Lou Gehrig’s disease, is caused by selective cell death in motor neurons in the brain and spinal cord. The mode of cell death in ALS motor neuron has been poorly characterized, which may prevent development of efficient therapy for the disease. Determination of cell death pathways involved in motor neuron cell death in ALS models should provide critical information for the development of ALS therapy approaches. The study is based on in vitro models of ALS including NSC-34 cell line (mouse motor neuron-like cell line) and iPS-MNs (ALS patient-derived induced pluripotent stem cells and their differentiated motor neurons) for cell death analysis. Discoveries from in vitro analysis will be confirmed in a mouse model of ALS.
To read more about Dr. Yang's research, view his faculty profile.
Yan H. Yu uses an interdisciplinary approach to examine brain plasticity for auditory and cognitive (language and music) processing in both neurotypical and neurologically impaired populations. In particular, Dr. Yu focuses on how experience (language and music) interacts with brain development starting from early infancy, and what’s the brain’s malleability in response to noninvasive brain stimulation. Dr. Yu’s research incorporates approaches from several disciplines spanning from communication sciences and disorders, cognitive neuroscience, neurolinguistics, psycholinguistics, bilingualism and neurointervention.
To view more about Dr. Yu’s research, review her faculty profile.
Read more about Dr. Yu's research on her faculty profile.
Molecular Neuroscience: Ion Channel Function and Regulation
Ion channels play crucial roles in all kinds of cells, especially excitable cells such as neuron and muscle cells. Dr. Yu’s lab uses the combination of molecular biology, biochemistry, biophysics, electrophysiology, and crystallography to study the molecular mechanism of the function and regulation of ion channels in physiological and pathologic conditions. Currently, the major focus in the lab is transient receptor potential (TRP) channels, a group of ion channels that play critical roles in sensory physiology. So far, TRP channels have been shown to be essential for the formation of sight, hearing, touch, smell, taste, temperature, and pain sensation. The NIH-funded research in Dr. Yu’s lab aims to shed light into the molecular mechanism of the function of ion channels in neurobiology and its relevance to neurological disorders.
To read more about Dr. Yu's research, view his faculty profile.
